WHAT ARE ESSENTIAL OILS?


Essential oils are natural aromatic extracts from plant material including grasses, leaves, flowers, needles, twigs, peels of fruit, seeds, bark, and roots. For example, rose essential oil comes from the flowers, basil from the leaves, lime from the rind, anise from the seeds, sandalwood from the wood, frankincense from the resin of its tree, and so on.
The Merriam-Webster dictionary defines essential oils as, “any of a class of volatile oils that give plants their characteristic odors and are used especially in perfumes and flavorings, and for aromatherapy”. The word “volatile” means a substance that easily evaporates at normal temperatures. These volatile oils given off by plants are responsible for the aromatic scents of flowers and other plant parts.
These aromatic essential oil compounds are stored in tiny pockets in plant material and must be extracted to be released. The type of plant material (leaves, flowers, roots) being used determines which method of extraction will produce the best results.
METHODS OF EXTRACTION
“True essential oils” are only extracted either by steam distillation or, in the case of citrus oils, expression.
Distillation
Steam distillation is the most popular method to extract essential oils.
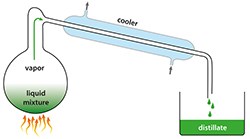

The steam must be hot enough to allow the release of the essential oil while not damaging the plant material. The steam with essential oil then passes through a cooling system where the steam condenses into a liquid consisting of essential oil and water. The essential oil, being lighter than the water, will float to the top and can then be separated from the water. The water by-product of distillation is called a hydrosol or floral water.


Expression


The expression method is used for extracting essential oils from the rinds of citrus fruits. Anyone who has peeled a fresh citrus fruit knows the refreshing, energizing aroma. But citrus oils do not hold up well when heat is used in extraction so they are usually obtained by a method called expression.
Expression is also known as the “expeller-pressed” or “cold-pressed” method of extraction since no heat is needed to extract the essential oil.
It is mostly used to extract citrus essential oils. In this process, the peels are pricked in order to puncture the cells containing the oils.
The peels are soaked in warm water and the oil is forced from the material under high mechanical pressure. Many base oils are extracted in a similar way.
Steam distillation and expression are the only two methods used to obtain “true essential oils”
However, some aromatic scents come from botanicals for which expression will not work and they cannot tolerate high-heat steam distillation.
As a result, the aromatic compounds of many flowers, such as neroli (orange blossom), jasmine, and rose are obtained using solvents.
Concretes & Absolutes:
Concretes and Absolutes are similar to essential oils since they are highly aromatic, concentrated extracts from plants. However, while essential oils must be produced by steam distillation or expression, concretes and absolutes require the use of a solvent for extraction and therefore cannot be called essential oils.
Fresh flowers or other plant materials are treated with a chemical solvent (see below). The “essential oils” of the plant material are dissolved by the solvent, the solvent is removed and what is left behind is a waxy aromatic compound called concrete.
The aromatic oils are extracted from the concrete using ethyl alcohol. Once the alcohol is removed, the remaining substance, called an absolute, is a very concentrated aromatic compound.
Types of Solvent Extraction:


This process utilizes petroleum solvents such as petroleum ether, hexane, or toluene; alcohol solvents such as methanol or ethanol; or carbon dioxide. As the solvent is added to the botanical it is absorbed and allows the release of the aromatic compounds.
These solvent extracted compounds are called “absolutes” and are very concentrated. Technically they should not be called essential oils. They are either extracted using:
- petroleum solvents (like hexane)
- ethanol solvents
- supercritical CO2 extraction process. This method helps to maintain the integrity of the base constituents of the oil and produces a highly concentrated finished product without using petroleum solvents.
Petroleum Solvent: A few years ago almost all absolutes, essential oils, and even carrier oils were extracted in this manner. However, since residues of the petroleum solvent, like hexane or benzene, may remain in the aromatic oil, solvent extraction was not recommended for essential oils used for aromatherapy. These residues may also cause allergic reactions and skin irritation.
Ethanol Solvent: While petroleum solvents can be very dangerous, ethanol (food grade drinking alcohol) is a rather benign solvent. The plant material is steeped in ethanol. The alcohol is evaporated out and a highly concentrated oil known as an absolute is left behind. Sometimes people will list an absolute, like vanilla, as an essential oil, but absolutes are not technically essential oils.
Supercritical Carbon Dioxide Extraction: Carbon dioxide extraction is steadily gaining popularity as a more natural method to extract essential oil from botanical 

It produces great essential oils but is also more expensive. Under pressure, the temperature of the carbon dioxide is increased to about 92 degrees Fahrenheit (33 degrees Celsius). At this temperature carbon dioxide enters a phase that is part liquid and part gas. Carbon Dioxide is an excellent solvent to extract pure essential oils since carbon dioxide does not chemically interact with the essential oil extracted. To remove the carbon dioxide solvent, simply decrease the pressure and the carbon dioxide returns to a gaseous state leaving the pure essential oil with no residues.
The Organic Rosemary Oil Extract we use as an antioxidant in some of our products is extracted using this Carbon Dioxide method. It is a method that can be used to obtain a USDA Certified Organic extraction.
